titanium protons|Titanium : Bacolod Titanium metal has some very valuable properties. In practice, it is pretty unreactive because, like aluminium, it forms a thin protective layer of the oxide, so it doesn't corrode. Its density is 4.5 grams per cm3, much less than iron, so titanium alloys are important in . Filipino words for setting include kapaligiran, tagpuan, tagpo, pinangyarihan, paligid, enggaste, lubog, nota, tugtugin and mga itlog na pinahahalimhiman. Find more Filipino words at wordhippo.com!
titanium protons,Titanium metal has some very valuable properties. In practice, it is pretty unreactive because, like aluminium, it forms a thin protective layer of the oxide, so it doesn't corrode. Its density is 4.5 grams per cm3, much less than iron, so titanium alloys are important in .
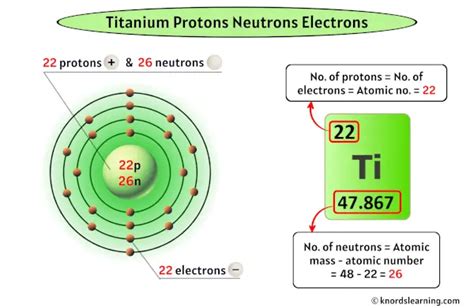
Learn about the number of protons, neutrons, electrons and electron configuration of titanium, a lustrous transition metal with a silver color and high strength. Find out .Titanium is the 22nd element of the periodic table so its atomic number is 22. The atomic number of an element is equal to the number of protons and electrons in that element. .
Titanium is the 22nd element in the periodic table and has a symbol of Ti and atomic number of 22. It has an atomic weight of 47.867 and a mass number of 48. Titanium .Atomic Number – Protons, Electrons and Neutrons in Titanium. Titanium is a chemical element with atomic number 22 which means there are 22 protons in its nucleus. Total .Titanium is a transition metal with the chemical symbol Ti and the atomic number 22. It has 22 protons and 22 electrons in its outermost shell. It is a light, silvery-white, hard, .Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver .Periodic table history. Identifiers. List of unique identifiers for Titanium in various chemical registry databases. Titanium is a chemical element of the periodic table with chemical .Atomic number: 22 (one titanium atom contains 22 protons, 22 electrons, and 26 neutrons). Relative atomic mass: 47.88.

Titanium - Periodic Table. 22. Ti. Group. 4. Period. 4. Block. d. Protons. Electrons. Neutrons. 22. 26. General Properties. Atomic Number. 22. Atomic Weight. 47.867. Mass . Titanium is a highly corrosion-resistant metal with great tensile strength. It is ninth in abundance for elements in the earth's crust. It has a relatively low density (about 60% that of iron). . Solvolysis can occur if ionisable protons are present in the ligand; 2NH 3-> TiCl 2 (NH 2) 2 + 2HCl 4H 2 O -> TiO 2.aq + 4HCl 2EtOH -> TiCl 2 (OEt .Number of Protons: 22: Number of Neutrons: 26: Number of Electrons: 22: Melting Point: 1660.0° C: Boiling Point: 3287.0° C: Density: 4.54 grams per cubic centimeter: Normal Phase: . Titanium has a low density and good strength. Titanium is the only element that burns in nitrogen. Titanium is used in fireworks. Common Uses: Mill products .
Titanium metal is considered to be non-toxic. As metal shavings, or powder, it is a considerable fire hazard. Titanium chlorides are corrosive. Characteristics: Pure titanium is a light, silvery-white, hard, lustrous .
Titanium is used in steel as an alloying element to reduce grain size and as a deoxidizer, and in stainless steel to reduce carbon content. Titanium has potential use in desalination plants for converting sea water into fresh water. Titanium is used in several everyday products such as drill bits, bicycles, golf clubs, watches and laptop computers.Protons/Electrons: 22. Neutrons: 26. Shell structure: 2,8,10,2. Electron configuration: [Ar]3d24s2. Oxidation state: 4. Crystal structure: Hexagonal. Titanium has a very high strength-to-weight ratio and is corrosion-resistant. Most titanium is used in the form of titanium dioxide (TiO 2 ). The number of neutrons can be found by subtracting the atomic number from its atomic mass. Number of Neutrons in Titanium = Atomic mass of Titanium – Atomic number of Titanium = 48 – 22 = 26. Number of Electrons in Titanium. For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom. Here’s how you can draw the Bohr model of titanium step by step. #1 Write protons, neutrons, and electrons of titanium atom. #2 Draw nucleus of titanium atom. #3 Draw 1 st electron shell. #4 Draw 2 nd electron shell. #5 Draw 3 rd electron shell. #6 Draw 4 th electron shell. Let’s break down each step in detail. Properties. Titanium has a melting point of 1660 +/- 10°C, boiling point of 3287°C, specific gravity of 4.54, with a valence of 2, 3, or 4. Pure titanium is a lustrous white metal with low density, high strength, and high corrosion resistance.titanium protons Titanium is a chemical element with atomic number 22 which means there are 22 protons and 22 electrons in the atomic structure. The chemical symbol for Titanium is Ti. Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine.Titanium (Ti) Titanium is the 22nd element in the periodic table and has a symbol of Ti and atomic number of 22. It has an atomic weight of 47.867 and a mass number of 48. Titanium has twenty-two protons and twenty-six neutrons in its nucleus, and twenty-two electrons in four shells. It is located in group four, period four and block d of the .All titanium atoms have 22 protons in their nuclei -- that’s what it means to be titanium. 76% of titanium atoms have 26 neutrons in them, and stable titanium atoms have between 24 and 28 neutrons. Adding neutrons to nucleus does not change its charge and so does not change the number of electrons orbiting around the nucleus. The chemical .
The structure of the titanium atom is complex, with 22 protons, 26 neutrons and 22 electrons. Creating a Bohr model of the atom is the best approach because, although it simplifies the nature of electrons, it does make the atomic structure easier to .
titanium protons Titanium How many protons, electrons, and neutrons are there in an isotope with the mass number of 81 and the atomic number of 35? a. 35 protons, 35 electrons, 46 neutrons b. 35 protons, 35 electrons, 116 neut; How many protons and electrons are in the lead isotope with 124 neutrons? An atom has 31 protons and 37 neutrons. titanium (Ti), chemical element, a silvery gray metal of Group 4 (IVb) of the periodic table. Titanium is a lightweight, high-strength, low-corrosion structural metal and is used in alloy form for parts in high-speed aircraft. A compound of titanium and oxygen was discovered (1791) by the English chemist and mineralogist William Gregor and .Titanium titanium (Ti), chemical element, a silvery gray metal of Group 4 (IVb) of the periodic table. Titanium is a lightweight, high-strength, low-corrosion structural metal and is used in alloy form for parts in high-speed aircraft. A compound of titanium and oxygen was discovered (1791) by the English chemist and mineralogist William Gregor and .Titanium is found in the middle of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. . The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope. Four artificial isotopes of titanium have also been . Basic Titanium Facts. Name: Titanium Atomic Number: 22 Element Symbol: Ti Group: 4 Period: 4 Block: d Element Family: Transition Metal Atomic Mass: 47.867(1) Electron Configuration: [Ar]3d 2 4s 2 Full: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2 (full). Discovery: William Gregor in 1791 Reverend William Gregor was an amature geologist .
Titanium. Element 22 of Periodic table is Titanium with atomic number 22, atomic weight 47.867. Titanium, symbol Ti, has a Simple Hexagonal structure and Silver color. Titanium is a Transition Metal element. It is part of group 4 (titanium family). Know everything about Titanium Facts, Physical Properties, Chemical Properties, Electronic .

Titanium is a chemical element with symbol Ti and atomic number 22. It is a lustrous transition metal with a silver colour, low density, and high strength. History and Discovery. Titanium was discovered by William Gregor in 1791 (Great Britain) [1]. Pure titanium was obtained after a long struggle of 119 years, by Matthew Hunter in 1910 .
titanium protons|Titanium
PH0 · Titanium Element Facts
PH1 · Titanium (Ti)
PH2 · Titanium
PH3 · Protons, Neutrons, Electrons for Titanium (Ti, Ti2+,Ti4+)